With the use of lithium battery, battery performance continues to decline, mainly for capacity attenuation, internal resistance increase, power decline, the change of battery internal resistance is affected by temperature, discharge depth and other conditions. Therefore, the factors affecting the internal resistance of the battery were discussed in combination with the structural design of the battery, the performance of the raw materials, the manufacturing process and the operating conditions.
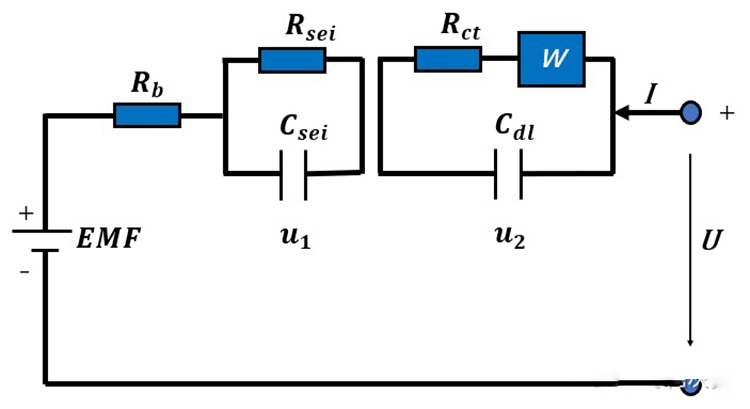
Resistance is the resistance of the current flowing through the battery when the lithium battery is working. (Aooser provided the internal resistance impedance tester )Generally, the internal resistance of lithium battery is divided into ohmic resistance and polarized resistance. Ohm internal resistance is composed of electrode material, electrolyte, separator resistance and contact resistance of each part. The internal resistance of polarization refers to the resistance caused by polarization during the electrochemical reaction, including the internal resistance of electrochemical polarization and the internal resistance of concentration polarization. The ohmic resistance of the battery is determined by the total conductivity of the battery and the polarization resistance of the battery is determined by the solid phase diffusion coefficient of lithium ion in the electrode active material.
Ohmic internal resistance is mainly divided into three parts, one is ionic impedance, two is electronic impedance, three is contact impedance. We hope that the internal resistance of lithium batteries is as small as possible, so we need to take specific measures to reduce the ohmic internal resistance of these three contents.
Ion impedance of lithium battery refers to the resistance of lithium ions to transfer inside the battery. In lithium batteries, the migration velocity of lithium ions plays an equally important role as the conduction velocity of electrons, and the ionic impedance is mainly affected by positive and negative electrode materials, separator and electrolyte. To reduce ionic impedance, you need to do the following:
Ensure positive and negative electrode materials and electrolyte have good wettability
In the design of the electrode plate need to select the appropriate compaction density, if the compaction density is too large, the electrolyte is not easy to infiltrate, will improve the ionic impedance. For the negative electrode sheet, if the SEI film formed on the surface of the living material is too thick during the first charge and discharge, the ionic impedance will also be improved, so it is necessary to adjust the formation process of the battery to solve the problem.
The electrolyte should have the appropriate concentration, viscosity and conductivity. When the viscosity of electrolyte is too high, it is not conducive to the infiltration between it and the active material of positive and negative electrode. At the same time, the electrolyte also needs a low concentration, too high concentration is not conducive to its flow infiltration. The conductivity of electrolyte is the most important factor affecting ionic impedance, which determines ionic migration.
The main influencing factors of separator on ionic impedance are: electrolyte distribution in separator, separator area, thickness, pore size, porosity and tortuous coefficient. For ceramic separators, it is also necessary to prevent ceramic particles from blocking the pores of the separator and preventing ions from passing through. In order to ensure that the electrolyte fully infiltrates the separator at the same time, there can be no residual electrolyte in it, reducing the use efficiency of the electrolyte.
There are many factors affecting electronic impedance, which can be improved from the aspects of material and technology.
Positive and negative electrode plates
The main factors affecting the electronic impedance of positive and negative electrode plates are the contact between living matter and collecting fluid, living matter itself, plate parameters, etc. The living material should be fully in contact with the collecting flow, which can be considered from the copper foil and aluminum foil substrate of the collecting fluid and the adhesion of the positive and negative slurry. The porosity of the living material itself, particle surface by-products, and uneven mixing with the conductive agent will cause the change of electronic impedance. Plate parameters such as density of living matter is too small, the particle gap is large, not conducive to electron conduction.
The influence factors of separator on electronic impedance include separator thickness, porosity and by-products in charge and discharge process. The first two are easy to understand. After the dismantling of the cell, it is often found that the separator is stained with a thick layer of brown material, which includes the graphite anode and its by-products, which will cause the blockage of the separator hole and reduce the battery life.
The material, thickness and width of the fluid collector, as well as its contact degree with the pole ear, all affect the electronic impedance. Fluid collection requires the selection of unoxidized passivated substrate, otherwise the impedance will be affected. Poor welding of aluminum foil to pole ear also affects electronic impedance.
The contact resistance is formed between the copper aluminum foil and the living material. It needs to pay attention to the adhesion of the positive and negative slurry.
The phenomenon that the electrode potential deviates from the equilibrium electrode potential when the current passes through the electrode is called electrode polarization. Polarization includes ohmic polarization, electrochemical polarization and concentration polarization. Polarization resistance refers to the internal resistance caused by the polarization of the positive electrode and the negative electrode of the battery during the electrochemical reaction. It can reflect the consistency of the battery internal, but due to the influence of operation and method, it is not suitable for production. The internal resistance of polarization is not constant and changes with time in the process of charge and discharge. This is because the composition of the active substance, the concentration and temperature of the electrolyte are constantly changing. Ohm internal resistance obeys Ohm's law, polarization internal resistance increases with the increase of current density, but the relationship is not linear. It usually increases linearly with logarithm of current density.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
