The safety problems of lithium-ion batteries still seriously restrict their large-scale application in the field of new energy vehicles and energy storage. In addition, with the development of lithium-ion batteries towards larger capacity, higher specific energy and faster charging ability, its safety issues will become more prominent. Research shows that the fundamental cause of lithium-ion battery combustion and explosion is the thermal runaway of the battery, and the condition of thermal runaway is temperature. When the battery temperature rises sharply due to abuse such as short circuit, overcharge and extrusion, Potential exothermic side reactions in the battery, such as the reaction between Electrolyte and bare lithium-embedded negative electrode caused by the decomposition of Solid Electrolyte Interface (SEI) on the negative electrode surface, the thermal decomposition reaction between positive electrode and electrolyte, and the exothermic reaction between binder and active material, were initiated successively and accelerated mutually. A large amount of hot and combustible gas accumulates inside the battery in a short time, resulting in thermal runaway behavior such as battery combustion or explosion. To date, various methods have been proposed to suppress thermal runaway; However, they have some inherent disadvantages, being either irreversible (single-use protection), using volatile and combustible electrolytes, or delayed thermal protection (140-150 ° C).
Based on this, have a professional related research papers published, content of the paper: by solvent evaporation method in a non-volatile, nonflammable and thermal reversible/ionic liquid gel polymer electrolyte, it is practical LiCoO2 | | Li battery to provide highly accurate and reversible thermal protection. At high temperatures, phase separation occurs in the gel electrolyte and polymer is deposited on the surface/diaphragm of the two electrodes, thus preventing Li+ migration and thus preventing thermal runaway. When the temperature is reduced to room temperature, the gel electrolyte recovers its original properties and the battery performance recovers. Notably, its thermal protection kicks in at 110°C, the critical temperature for thermal runaway. More importantly, this thermal protection process can be repeated many times without affecting battery performance, showing extraordinary thermal reversibility. Such precise and reversible protective effects have never been reported in any electrolyte system, and this work opens an exciting avenue for the safe operation of high-energy lithium batteries, according to the report.
Electrolyte is an important part of the battery, the role of electrolyte is like "blood", the main role is to conduct lithium ions between the positive and negative electrodes, its performance affects the energy density of lithium ion batteries, high and low temperature performance, cycle life and safety performance. At present, the flash point of linear carbonate solvent is low (less than 35℃). Under the condition of high temperature and pressure inside the battery, once the heat is out of control, the electrolyte is very easy to ignite. Therefore, the development of flame retardant and high flash point electrolyte formula is one of the most effective ways to prevent spontaneous combustion of lithium ion batteries. Therefore, the electrolytic system designed by professionals is (0.5M LiTFSI-5wt% PBMA/[EMIM][TFSI]). The reason why 1-ethyl-3-methylimidazole bis (trifluoromethane sulfonyl) imide ([EMIM][TFSI]) ionic liquid solvent is chosen here is because of its low viscosity and high electrical conductivity. Lithium bifluoromethane sulfonyl imide (LiTFSI) was chosen because of its high solubility. The polymer is poly (benzyl methacrylate) (PBMA), whose low critical solution temperature (LCST) is approximately 110℃, below which the components of the mixture are miscible in all ratios. The preparation of the polymer/ionic liquid gel electrolyte is based on a co-solvent evaporation method in which the tetrahydrofuran co-solvent contributes to the dissolution of the material.
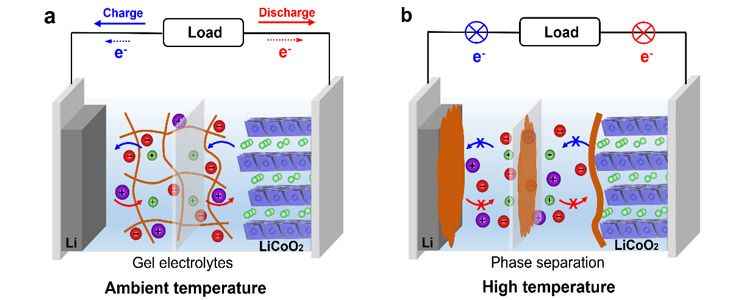
Figure 1. Working mechanism of the temperature-sensitive "switch" electrolyte: (a) At ambient temperature, the gel electrolyte supports lithium ion conduction and battery operation; (b) At high temperature, phase separation occurs in the gel electrolyte and the polymer is deposited on the electrode surface, thus blocking Li+ conduction and terminating the charge-discharge reaction of the battery.
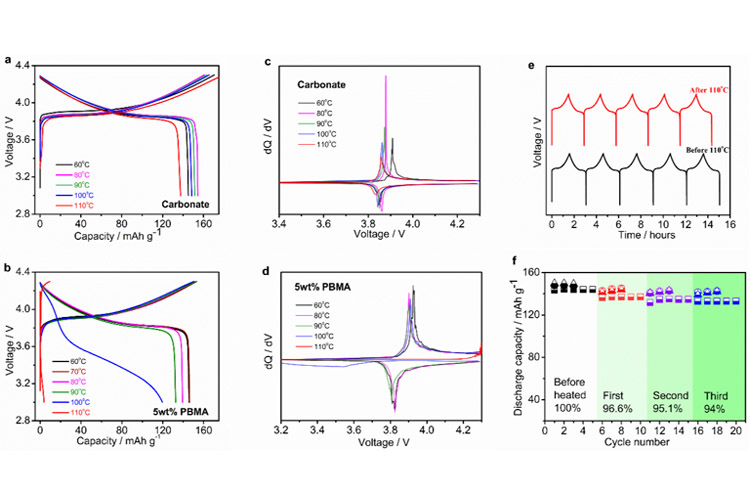
FIG. 2. Using commercial carbonate electrolyte and self-protection gel electrolyte LiCoO2 | | Li battery charge and discharge with the temperature change curve and the corresponding dQ/dV curve (a, c) commercial carbonate electrolyte; (b, d) Self-protecting gel Electrolyte 0.5M LiTFSI-5wt% PBMA/[EMIM][TFSI]; (e, f) hot self-protection electrolyte reversibility test, LiCoO2 | | Li battery charge and discharge curves before and after 110 ℃ and discharge specific capacity comparison.
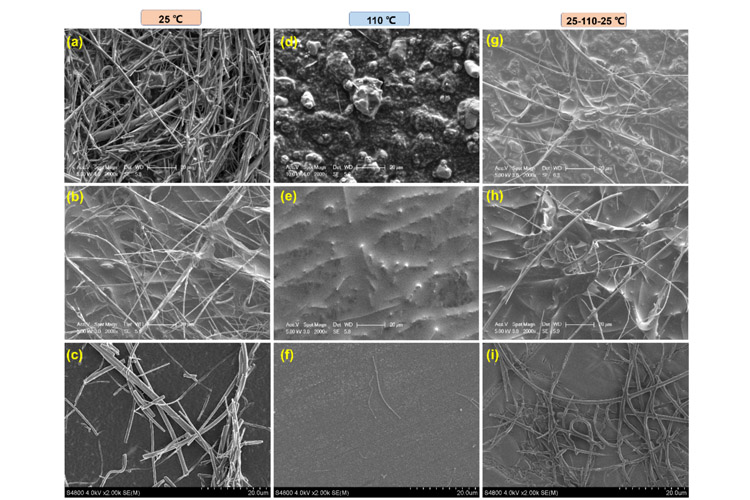
FIG. 3 SEM images of each component of the self-protection electrolyte battery after running at 25℃, 110℃, 25-110-25℃ : (a-c) LiCoO2 positive electrode, (d-f) Li negative electrode, (g-i) diaphragm.
Has a professional staff developed a volatile, flammable, thermal reversible LiTFSI PBMA / [EMIM] + [TFSI] gel electrolyte, it is of practical LiCoO2 | | Li battery provides a highly accurate and reversible thermal protection effect. Due to the phase transition of the gel electrolyte, the PBMA polymer will precipitate and deposit uniformly on the electrode surface and diaphragm at 110℃, thus preventing ion migration and effectively preventing thermal runaway of the battery. When the temperature returns to room temperature, the gel electrolyte recovers its original properties and the battery can continue to work normally. Of concern, the thermal protection is activated at 110℃, which is the critical temperature for thermal runaway. More importantly, this thermal protection process can be repeated many times without affecting battery performance, showing extraordinary thermal reversibility. It is reported that such precise and reversible protective effects have never been reported in any electrolyte system, and this work provides new ideas for the development of reversible temperature-sensitive and intelligent temperature-controlled lithium-ion batteries.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
