Aging generally refers to the completion of battery assembly liquid injection, after the first charging and discharging formation, can have room temperature aging or high temperature aging, in the previous article "The impact of Lithium battery aging system on battery performance" mentioned that the purpose of aging is mainly the following aspects:
1. The battery is placed at high temperature or normal temperature for a period of time, can ensure that the electrolyte can be fully infiltrated on the pole plate, conducive to the stability of the battery performance;
2. After the pre-formation process of the battery, the negative graphite electrode inside the battery will form a certain amount of SEI film, but the membrane structure is tight and the pores are small. The aging of the battery at high temperature will help to restructure the SEI structure and form a loose porous film.
3. After formation, the voltage of the battery is in an unstable stage. After aging, the active substances in the positive and negative electrode materials can accelerate some side effects, such as gas production and electrolyte decomposition, so that the electrochemical performance of the lithium battery can quickly reach stability.
4. Eliminate serious self-discharge unqualified batteries, easy to screen high consistency of the battery.
Among them, aging process screening internal micro - short circuit cell is a main purpose. During the battery storage process, the open-circuit voltage will decrease, but the amplitude is not very large. If the open-circuit voltage decreases too fast or too large, it is an abnormal phenomenon. Battery self-discharge can be divided into physical self-discharge and chemical self-discharge according to different reaction types. Considering the effect of self-discharge on battery, self-discharge can be divided into two kinds: the self-discharge with reversible capacity loss and the self-discharge with permanent capacity loss. Generally speaking, the energy loss caused by physical self-discharge is recoverable, while the energy loss caused by chemical self-discharge is basically irreversible. Battery self-discharge comes from two aspects: (1) self-discharge caused by chemical system itself; This part is mainly due to the side reaction in the battery, including the change of positive and negative electrode material surface film; Potential changes caused by electrode thermodynamic instability; Dissolution and precipitation of metal foreign matter; (2) The micro short circuit inside the battery caused by the separator between the positive and negative electrodes leads to the self-discharge of the battery.
When lithium ion battery is aging, the change of K value (voltage drop) is the formation and stabilization process of SEI film on the surface of electrode material. If the voltage drop is too large, it indicates that there is a micro short circuit inside, so the battery can be judged as unqualified product. K value is a physical quantity used to describe the self-discharge rate of a cell. It is calculated by dividing the open-circuit voltage difference of two tests by the time interval △t of two voltage tests. The formula is: K = (OCV2-OCV1) /△t.
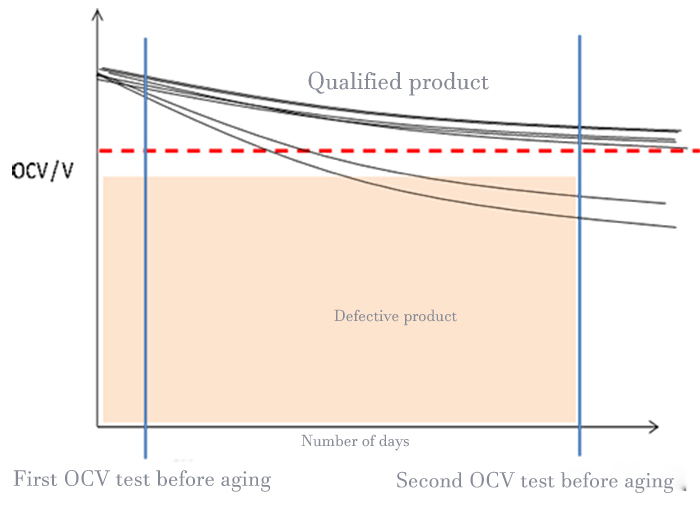
The particles or trace metal residue on the pole plate, the tiny defects on the separator, and the dust introduced in the assembly process of the cell will cause the micro short circuit inside the cell. For micro-short-circuit cells, it is impossible to complete the screening only by capacity and primary voltage, so the K-value test must be introduced to determine whether the cell has micro-short-circuit by accurately calculating its voltage drop rate, as shown in Figure 1.
The basic principle of the battery internal short circuit caused by metal foreign matter is divided into two processes. Larger metal particles directly Pierce the , causing a short circuit between the positive and negative electrodes, which is a physical short circuit. In addition, when the metal foreign matter is mixed into the positive electrode, the positive electrode potential rises after charging, the metal foreign matter dissolves at high potential and diffuses through the electrolyte, and then the dissolved metal at low negative potential precipitates and accumulates on the surface of the negative electrode, finally penetrating the and forming a short circuit, which is a chemical dissolution short circuit. The most common metal foreign bodies in battery factories are Fe, Cu, Zn, Al, Sn, and SUS.
In the face of such complex metal foreign matter, measures are often taken at the manufacturing site to prevent foreign matter from mixing into battery products. Such as electrode paste with electromagnetic iron removal equipment to remove metal impurities such as Fe, pole slice cutting or die cutting process with brush to clean cutting burr, pole ear or coating edge stick tape protection, easy to produce metal chips process (welding) with dust collector adsorption foreign matter, etc. During the process detection, internal short-circuit unqualified products are detected by voltage resistance test before liquid injection. The aging process detects nonconforming products by the battery voltage drop ΔV.
The voltage drop K is a function of time t, charging state and temperature T. Therefore, there are three main process parameters of aging process: (1) charging state of aging battery, (2) aging storage temperature, and (3) aging time.
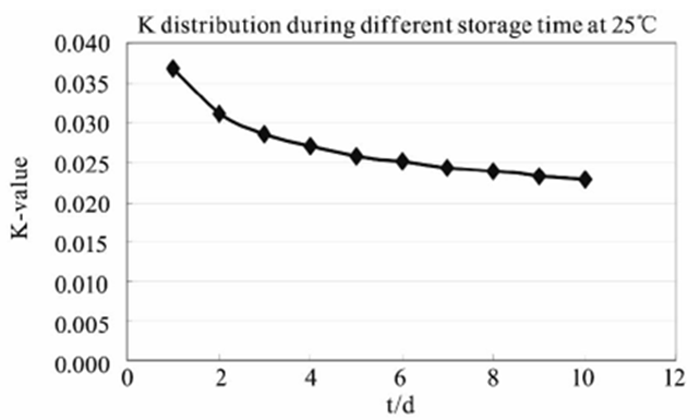
Under certain temperature conditions, the relation curve between K and time is shown in Figure 4. When the temperature is constant, K decreases with the increase of the standing time. This simply means that the self-discharge rate of the battery will decrease with the extension of time, but the size of the self-discharge in a given time is certain, which does not improve the self-discharge in nature.
Under the condition of a certain storage time, K value increases with the increase of temperature. With the increase of temperature, the activity of the system increases, the reaction rate is accelerated, the loss of active lithium is accelerated, and some side reactions are even produced. The dissolution of metal impurities in the positive electrode and precipitation in the negative electrode will also accelerate with the increase of temperature. Because it takes a long time for the battery's internal micro short circuit to show up. Therefore, high temperature aging can accelerate the process of selecting unqualified products, saving time and production costs.
Under certain conditions of storage time and storage temperature, K value increases with the increase of charging state in a certain voltage range (3.8-4.2V). With the increase of SOC, the self-discharge rate of the battery will be accelerated, and the interface impedance of the negative electrode will increase with the increase of storage SOC. According to the chemical equilibrium, the negative electrode will consume more active Li as the concentration of Li increases gradually and the interfacial reaction moves towards the direction of Li consumption.
The general aging procedure is: charge to 4.0-4.2V, store at room temperature for 7d, and store at high temperature for 45℃ for 7d, detect the voltage difference before and after aging of the battery and remove unqualified products. The battery is left open at high temperature or normal temperature for 7 days or 28 days, and the self-discharge performance is determined by measuring the discharge quantity to the cut-off voltage of the battery. This method requires a one-month shelving test on the battery, which has a long time cycle, large influence factors and low accuracy. Moreover, it occupies more equipment and sites for a long time, and the test safety is poor, which is a large waste of manpower and financial resources. Pierrot S. Attidekou from the University of Newcastle, UK, has reduced the screening time of self-discharge of lithium-ion batteries from weeks to less than 10 minutes by using AC impedance. With further optimization, the screening time is expected to be further reduced to 1 minute.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
