At present, the need of high-endurance new energy vehicles forces the energy density of batteries to become higher and higher, and the use of thick electrodes with high load density active materials is one of the most practical strategies. However, their long cycle use process is accompanied by serious attenuation of electrochemical performance, power performance is not satisfied, and the capacity retention rate is getting worse and worse. So what exactly is causing the bottleneck of poor performance?
Kyu-Young Park et al. explored the key processes that restrict battery decay by designing thick electrodes with different area degrees.
1. Experimental design
Using NCM622: carbon black: PVDF 97:1.5:1.5 ratio and NMP mixed into pulp, after coating, drying and roller pressing, two kinds of electrodynamic half cells (2032) with different surface densities (20 and 28mg/cm-2) were prepared, and the pressure was between 2.8 and 2.9, in order to ensure better porosity. The charge and discharge cycle of the multi-channel device was carried out with the charge and discharge interval of 2.8-4.3V and the rate 1C was about 150mA/g. EIS, chemical composition and morphology were analyzed after every 20 cycles.
2. Results and discussion
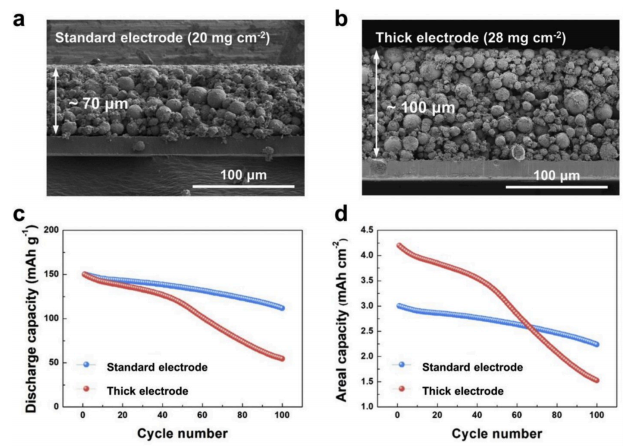
The following is the cross-section diagram of the electrodes of two thickths, respectively 70 and 100μm(standard electrode, thick electrode), the rest of the porosity, 1C current density and other design parameters are basically the same, and then the 1C cycle test is carried out. It is found in Figure c that although the capacity of the thick electrode of 100μm is only 40% higher than that of 70μm, but after 100 battery cycles, The thick electrode has a capacity retention rate of only 36%, while the standard electrode has a capacity retention rate of 76%. Even taking into account the volume specific capacity, the thick electrode after attenuation in Figure c is still much lower than the electrode. Interestingly, in Figure c, even in the initial cycle process, the circulation curves of the thick electrode and the standard electrode are close, and the attenuation degree is similar. Thick electrodes are getting worse.
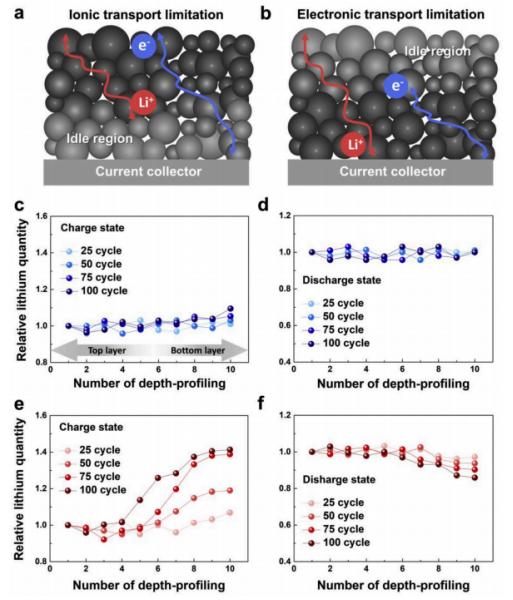
In illustrating the poor electrochemical performance observed, the authors note that thick electrodes may be subject to kinetic limitations caused by how fast or slow charge carriers migrate, which in electrochemical processes is either controlled by lithium-ion transport or by the transport of electrons that accumulate along the electrode. And, in each case, assuming that the main source of supply of electrons and lithium ions to the electrode is carried out from the electrode/collector interface and the electrode/electrolyte interface, in each case there will be a clear spatial distribution of both after the reaction.
In Figure a, it is explained that the slow migration of lithium ions determines the reaction rate. On the side near the fluid collector, the electrochemical reaction rate in this region is low because long-distance lithium ions cannot migrate quickly, while on the side near the electrode liquid, both lithium ions and electrons maintain a high concentration, so the reaction can be carried out well. In Figure b, it is explained that the slow electron migration determines the reaction rate. On the side close to the fluid collector, the electrochemical reaction rate in this region is higher because lithium ions can migrate over, while on the side near the electrode liquid, the electron migration is blocked and the presence concentration is lower, the reaction rate becomes worse. The darker the color in the figure, the better the reaction is. In order to verify the two guesses, the author characterized the SOC state characteristics under different electrode thicknesses during the reaction process and the charging and discharging process. Figures c and d show the test every 25 cycles during the charging and discharging process of the standard electrode. The larger the X-axis coordinate is, the closer the distance from the fluid collector is. The amount of lithium embedded in SOC at different locations is almost the same, indicating that even at 1C ratio, the electrochemical reaction is uniform, while in Figure e, at 25 cycles, the electrode SOC is relatively uniform. After 50 cycles, lithium ions are diffused from the positive electrode to the negative electrode during the battery charging process, and the differences at different locations are increasingly large, which is also consistent with the previous cycle retention curve. In Figure f, it is found that the difference is not particularly obvious in the discharge process of thick electrodes. The author believes that this is because the charging process in the early stage leads to more lithium inlay near the fluid collector side, while there are fewer lithium ions near the electrolyte separator. Therefore, the concentration of lithium ions at the separator is higher during the discharge process. It will not show too much difference, from the SOC characteristics of thick electrodes, it can be analyzed that slow lithium ion migration is an important reason for capacity attenuation.
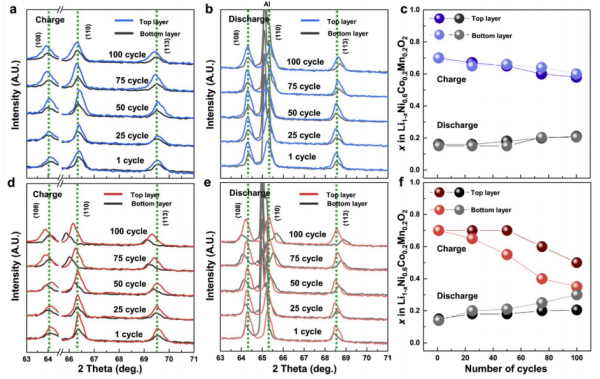
The XRD characterization also verified the speculation of uneven distribution of lithium inlay. As can be seen from the standard electrodes in Figure a and b, there was no significant difference between the XRD peaks at the bottom and top after different cycles. The supporting data showed that after 100 cycles, SOC at different locations differed by up to 5%, while serious peak position shifts were observed at the bottom and top in d and f.
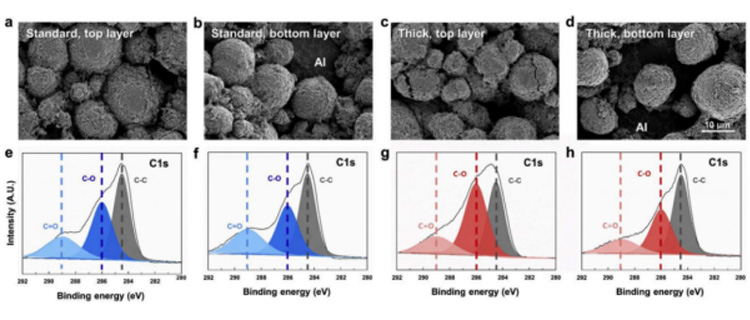
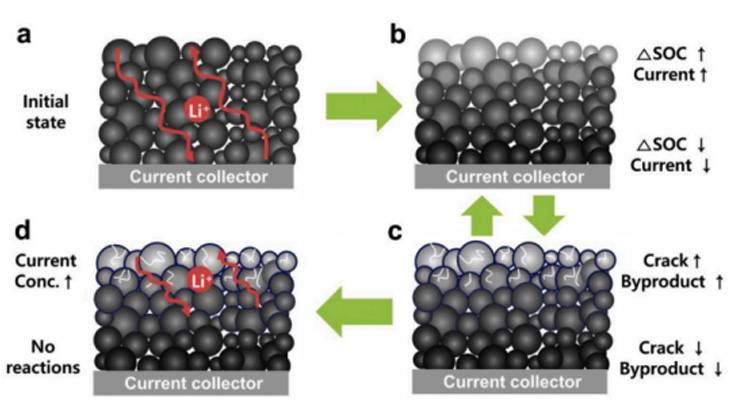
Due to the uneven SOC caused by material transport, the electrode at the top of the separator will have a relatively high proportion of electrochemical reaction, while the reaction near the bottom of the fluid collection is poor. With the progress of the cycle, the thick electrode will show worse and worse performance, and the reaction current density at the top position will become larger and larger, forming a current hot spot, which will destroy the active material. Causing cracks or irreversible phase transitions, the high current density will also form a solid phase lithium ion concentration distribution with a large gradient in the secondary particles, resulting in a stress field, leading to particle breakage, exposing the new interface, side reactions, forming a thick organic layer at the new interface, causing an increase in impedance, and thus becoming a failure dive of the battery. The above explanation can be verified from the thick electrodes c, g and d, h in the figure below, where the content of C=O is increased.
Based on the above work, a mechanism model of thick electrode attenuation of batteries is proposed.
a: In the initial cycle process, lithium ions can diffuse to the fluid collecting side in the entire electrode distribution, and the electrochemical reaction is relatively uniform, and there is no large lithium ion concentration gradient distribution;
b: As the cycle progresses, the limitation of lithium ion transport begins to cause an uneven electrochemical reaction in the upper and lower parts, meaning that voltage drop IR and concentration polarization become increasingly important in thick electrodes, and subsequently, the active particles in the upper part are subjected to higher effective current densities.
c: The high current density leads to the rupture of active particles, exposing the new interface, which in turn leads to the increase of the porosity of the pole sheet, and the migration of lithium ions to the fluid collecting side becomes more difficult, resulting in higher current density occurring at the upper particles, and the particles are more broken, etc., forming a negative feedback.
d: Seriously, the electrochemical reaction occurs only at the upper part, and the current hot spots are formed, which can lead to great risks in battery management.
The authors also verified that for the same standard electrode and thick electrode, the CCCV method can significantly reduce the SOC difference of the thick electrode at the same current density.
3. Conclusion
By using batteries designed with different electrode thickness, the authors verify that lithium ion diffusion is the limiting factor of charge transfer, but not electron transfer. This is also the reason why SOC at different locations is uneven, voltage drop IR increases, particle breakage and even battery diving under charge and discharge in batteries designed with thick electrodes. The electrode plate is designed according to the ion transport characteristics to avoid the phenomenon of excessive local current density, so as to improve the battery life.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
