In recent years, with the increasing market demand of lithium battery in new energy vehicles and energy storage, the output of electrolyte of our country is increasing. But at the same time, the continuous development and iteration of the new technology and process of lithium battery also demand higher requirements for the upgrading applicability of electrolyte in the aspects of high nickel, high pressure, high specific energy and high safety.
For example, high nickel cathode material has become the mainstream technology route of the current high energy density lithium battery. With the increasing proportion of nickel and the use of silicon carbon anode, the development and production of electrolyte has brought new challenges.
At the same time,In order to alleviate range anxiety, lithium-ion batteries are increasing their energy density, and thus their voltage. The higher the voltage is, the stronger the decomposition ability of the electrolyte will be, which may adversely affect the performance and safety of the lithium battery.
In addition, the storage performance, cycling performance and safety performance of lithium battery under high temperature, fast charging and other environments are closely related to the performance of the electrolyte.
The above industry pain points are more complex to solve, and the technical threshold is high. In the face of the development trend of new technology, it is necessary to tackle the key issues related to electrolyte in order to meet the new requirements of new material system for electrolyte.
In recent years, new high-voltage cathode materials such as lithium nickel manganate and layered Li-Mangan-based materials have been gradually developed, whose discharge voltage can reach more than 5V, but they have not been commercialized yet. The most important reason is that the operating voltage of the current commercial electrolyte cannot be matched.
Current researches on electrolyte mainly take LiPF6 as lithium salt and single or mixed carbonate solvent as the main solvent, including EC, DMC, EMC, DEC and PC. When the operating voltage is greater than 4.3V, the decomposition of conventional electrolytes usually occurs. This is due to the instability of common organic carbonates such as chain carbonates DMC, EMC, DEC, and cyclic carbonates PC and EC at high voltages.
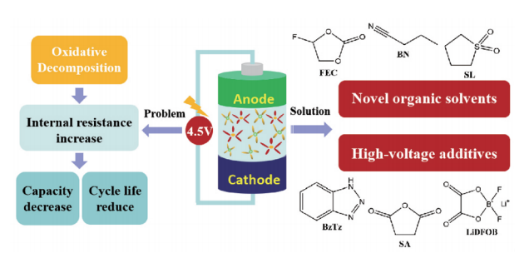
The problem of traditional electrolyte under high voltage and the solution of increasing the working voltage of electrolyte:
At present, more research is to use high voltage materials to improve the working voltage of the electrolyte, including the use of high pressure solvent, high pressure additives and high concentration lithium salt electrolyte.
High-pressure solvents mainly include fluorinated solvents, sulfone solvents, nitrile solvents, ionic liquids, etc. Compared with high-pressure solvents, high-pressure additives are more popular because of their small amount, obvious effect and low cost. At present, the main additives studied more are boron additives, benzene derivatives and heterocyclic additives, phosphite additives, etc. In addition, ether, sulfur, organosilicon, ionic liquid and other additives are also widely studied and applied. High concentration lithium salt electrolyte refers to the electrolyte with the concentration of lithium salt above 3mol/L. This electrolyte has excellent oxidation/reduction resistance, can prevent the corrosion of aluminum collector fluid, and high rate performance of battery. At present, the lithium salt with the highest degree of commercial application is LiPF6, which is easy to decompose and produce HF in the presence of trace water or high temperature, destroying the electrode structure and failing to meet the requirements of high pressure and high safety electrolyte, so it needs to be modified. New lithium salts such as LiFSI and LiTFSI have attracted much attention due to their good thermal stability and insensitivity to water.
For power battery, fast charging is also an important direction of electrolyte development.
The performance of high power electrolyte of lithium battery is mainly studied in two aspects: first, the charge migration impedance of SEI film increases under high rate charging, which increases the electrode polarization during charging. Thirdly, under the condition of high rate charging, lithium evolution is easy to occur in the late period of constant current charging of lithium battery, leading to deterioration of SEI film condition and deterioration of battery performance. Therefore, firstly, through lithium salt optimization, adding lithium salt conducive to high rate charge and discharge can improve the high power performance of the battery to a certain extent; Second, by adding high-power performance additives, by adding film forming additives with better effect than EC to reduce the electrode interface charge transfer impedance under high rate charge and discharge, or adding lithium salt deposition improver to prevent the growth of lithium branching crystals during high rate charge, improve the high-power performance of the battery.
Multi-application scenarios are the driving force behind the development of lithium batteries as well as their advantages. In electric vehicles, portable electronic devices and other applications, lithium batteries only need to meet the operating temperature of 15 to 35°C. However, some special applications require lithium batteries to break through this temperature range.
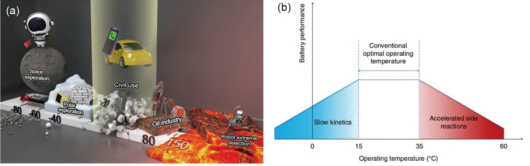
(a)Schematic diagram of different temperature application scenarios faced by lithium batteries; (b) The influence of extreme operating temperature on batteries
Too low working temperature will slow down the kinetic process of electrochemical reaction in lithium battery. Due to the increase of electrolyte viscosity, the decrease of electrolyte salt solubility, and the increase of Li+ desolvation energy barrier, at low temperature, the rate of charge transfer from ion diffusion in electrolyte and electrode material to the electrode - electrolyte interface significantly decreases at each stage of charge transfer, which will cause the increase of polarization and capacity of the battery, and even difficult to work. In addition, too low working temperature may also change the electrochemical reaction path. For example, at low temperature, Li+ which should be embedded between the negative graphite layers may be reduced on the surface of the negative graphite electrode, forming dendrites and endangering the safety of the battery. However, in high temperature working environment, due to the deterioration of electrolyte/electrode interface stability, the occurrence of electrode and electrolyte side reactions cannot be prevented, so the main challenge faced by lithium batteries is excessive side reactions. In addition, high working temperature also challenges the thermal stability of electrode materials.
When the local temperature of the lithium battery system rises, the reaction inside the battery is out of control, and the thermal contraction of the diaphragm further leads to internal short circuit and releases more heat, resulting in the complete thermal control of the lithium battery.
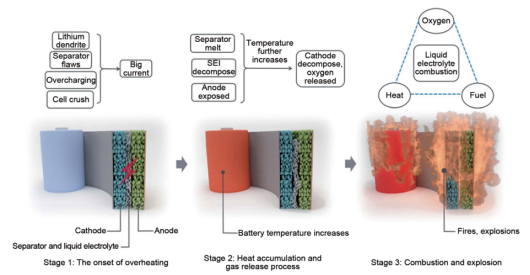
The three stages of the thermal runaway process of a lithium battery:
In the review of the Research Progress of intelligent safety Electrolyte in lithium-ion batteries published by Tsinghua University Ou Yu et al., the research progress of intelligent safety electrolyte in lithium-ion batteries was summarized from three aspects in view of different abuse conditions of batteries (thermal abuse, mechanical abuse, overcharge) : Thermal response polymer electrolyte to prevent thermal abuse, shear thickening electrolyte to prevent mechanical abuse, and REDOX shuttle to prevent electrical abuse.
The mechanism of action of thermoresponsive polymer electrolytes is mostly based on the principle of phase transition, which is a reversible phase transition from hydrophobic to hydrophilic. When the temperature rises beyond the critical value, the polymer molecular conformation changes reversibly. This conformational change leads to a decrease in the ionic conductivity of the polymer itself and an increase in the charge transfer impedance of the lithium battery, thus preventing thermal runaway from occurring. When the temperature is lowered, the thermosensitive polymer can be redissolved in the electrolyte, restoring its ionic conductivity. In ionic liquids, thermal responsive polymers such as polyethylene oxide (PEO), polybenzyl methacrylate (PBMA) and polyn-butyl methacrylate all have low critical solution temperatures between 80 and 200℃, completely covering the thermal uncontrolled temperature of lithium batteries, and can be used in lithium batteries.
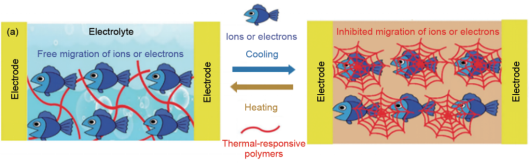
Inhibition of ion or electron conduction between electrodes by reversible thermal response polymers prevents thermal runaway in electrochemical storage devices
At present, the recycling of lithium batteries, especially the recycling of power batteries, is still difficult, so improving the cycle life of batteries is a way to alleviate this situation.
Dr. Lu Di from Zhejiang University proposed a self-purifying electrolyte. 1.6M lithium difluorosulfonimide (LiFSI) was dissolved in functional nitrile solvent 3-(triethoxysilane) prolonitrile (TEOSCN). TEOSCN can effectively remove active harmful substances in the electrolyte. FSI- can form SEI/CEI with lower interfacial impedance at graphite and Ni-rich positive electrodes at lower concentrations. The self-purification electrolyte has realized the MCMB | | NMC811 battery stable long cycle, all under 25 ℃ circulating capacity remain at a rate of 91% after 1000 laps, under 60 ℃ circulating capacity remain at a rate of 81% after 500 laps. Even if the electrolyte is exposed to the air after 1 hour, the electrolyte can still maintain the MCMB | | NMC811 full battery stable loop, which greatly simplifies the production process of the lithium ion battery and cost. This effective self-purification strategy will stimulate more research on electrolytes with unique physicochemical properties, and provide a promising approach for the development of the next generation of high-specific energy, long-life lithium batteries.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
