Although fluoride has become a common electrolyte, solvent and additive for high-performance lithium/sodium ion batteries, there are still some problems with fluoride.
1. Overview of fluorine
In lithium-ion batteries and sodium-ion batteries, electrolyte salts containing highly fluorinated counter ions are usually used. LiPF6 is a typical example, where the electron-absorbing effect of fluorine helps to distribute negative charge, thus reducing the lattice energy of salt, improving ion dissociation, and promoting the dissolution of salt in organic solvents. More important is the role of hexafluorophosphate anions in passivation: 1) SEI formation. In the SEI layer, SEI components such as LiF/NaF, fluorophosphate and fluorinated organic matter are derived from the decomposition of fluorinated anions and fluorinated solvents and electrolyte additives. 2) The ability to blunt aluminum to prevent the corrosion of positive collector fluid at high potential. In addition, the use of highly fluorinated solvents may also increase the non-flammability of the electrolyte, especially in lithium metal batteries, where fluorinated solvents are conducive to the formation of highly fluorinated, Lif-rich SEI. When 1-vinyl fluoride carbonate (FEC) replaces EC as the co-solvent of dimethyl carbonate (DMC) in the electrolyte, lithium metal can be deposited/dissolved at higher rates, achieving higher stability and longer life (Figure 1).
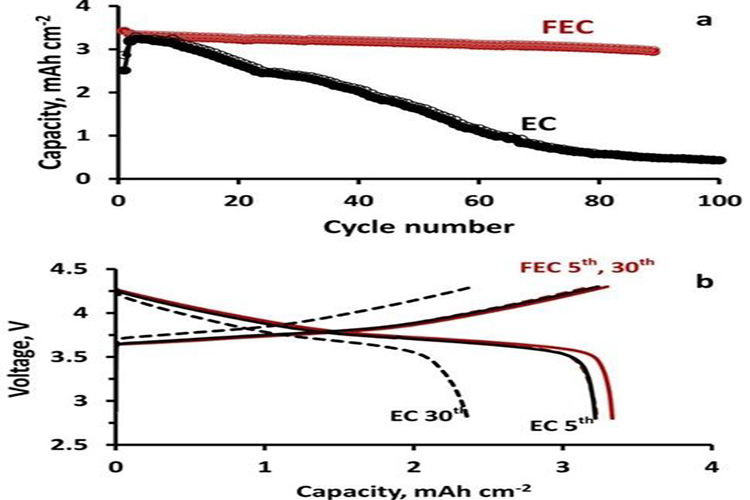
Figure 1 USES 1 M LiPF6 FEC/DMC and EC/DMC in the Li | | NMC622 performance of the battery.
Fluoride solvent also have an impact on the positive side, for example, in the SiO | | NMC622 cells make TFEP, FEMC and HFE, And the graphite | | Li1.2 Mn0.56 Co0.08 Ni0.16 O2 using FEC, 1, 2 - two fluorine ethylene carbonate, and 1,1,2,2 - ethyl - 2,2,3,3 four fluorine - four fluorine propyl ether are to strengthen the long cycle of the battery performance and stability of CEI. In sodium-electric systems, fluorinated electrolytes perform a similar function.
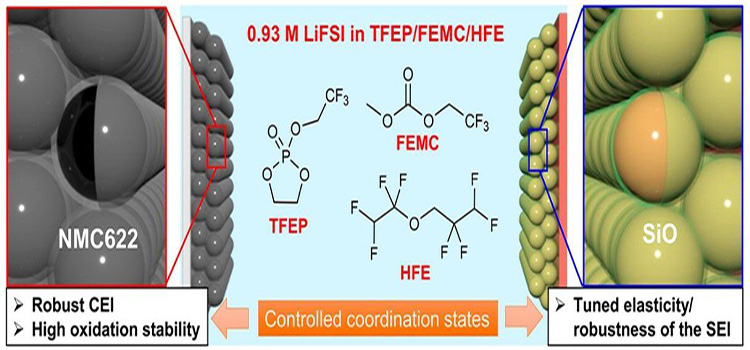
Figure 2 in the SiO | | NMC622 LiFSI in batteries and the effect of fluoride solvent electrolytes.
It can be found that FEC frequently appears in these literature. FEC is especially suitable for use in batteries with lithium, sodium and silicon as negative electrodes, because these materials are facing huge volume expansion. FEC forms SEI rich in LiF, Li2O and fluorinated organic matter through defluorination and rapid interfacial reduction. In contrast to the ethylene carbonate (VC) additive, the SEI layer formed by FEC on the Li metal contains the structure LiF nanocrystals, which can improve the Li+ conductivity and make the battery suitable for high power applications.
In addition to FEC, there are several other fluorinated electrolyte additives that have a positive effect on battery circulation. Such as lithium difluoride (oxalate) lithium borate (LiDFOB), difluoride and trifluorinated acetic anhydride and fluorophosphate, these fluoride additives can achieve stable deposition of metal Li by stabilizing SEI.
2. Problems with fluorine
Although fluoride shows a variety of advantages in batteries, some problems cannot be ignored, and FEC is perhaps the best illustration of this. For example, FEC cannot form SEI films as well on graphite anode as it does on lithium metal. When using high pressure cathode materials such as LiNi0.5Mn1.5O4(LNMO), high FEC concentration will lead to poor cycling performance. However, in some sodium battery systems using hard carbon cathode, the non-fluorinated organic carbonate electrolyte can form better, highly conductive SEI. At the same time, FEC is unstable and will be continuously consumed and degraded in the cycle. Even at high temperature, FEC will lose its function (FIG. 3, 4), and some toxic and corrosive impurities will be produced after FEC degradation.
Fluorinated electrolyte components are also a concern when recycling used batteries, as they can cause significant safety and environmental hazards during handling and disposal, releasing toxic HF and PF5. Designing fluorine-free electrolytes would therefore be an important step towards creating more recyclable batteries.
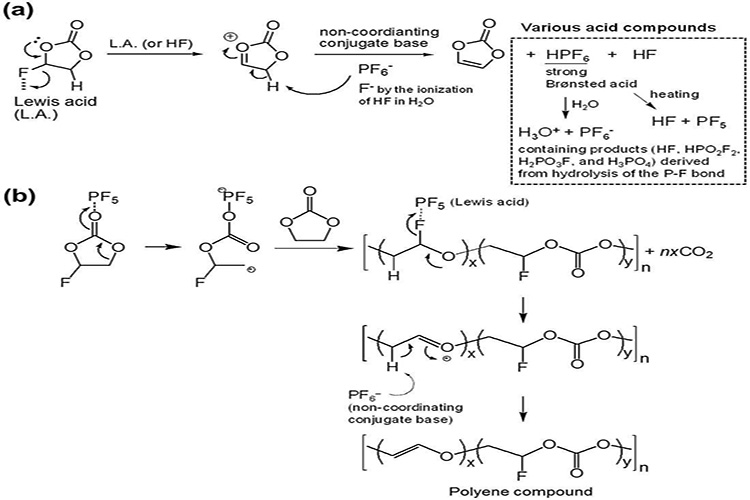
Figure 3 Cascade reaction caused by binding degradation of FEC and LiPF6.
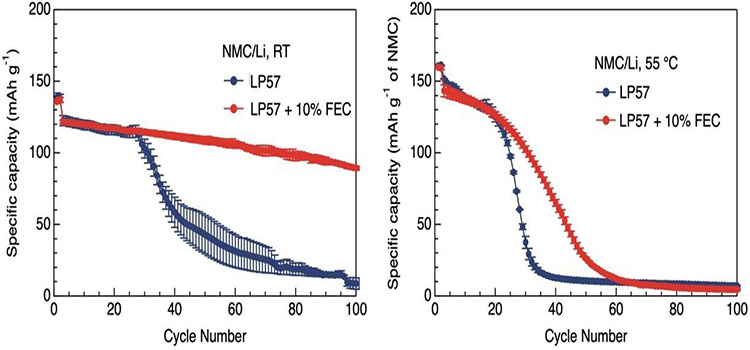
Figure 4 at room temperature (left) and 55 ℃ (right), including and excluding 10% of FEC LP57 electrolyte Li | | NMC111 loop performance of the battery.
3. Alternatives to fluorine
The development of non-fluorinated anions to replace PF6− has become an important task for the development of fluorine-free electrolyte systems. This fluorine-free anion needs to meet a variety of requirements, the most important being ionic conductivity. Dissolution of electrolyte salts in non-aqueous solvents involves two steps :(i) dissociation of cations (Li+ or Na+) from anions, and (ii) formation of coordination structures between cations and solvent molecules. The properties of anions have a great influence on the first step, so the focus of the research is to design salts with weak coordination anions. Many of these non-fluoridated salts have become competitive with electrolytes of LiPF6/ NAPf6-carbonate systems in ionic conductivity (meaning ionic conductivity in the range of 1-20 mS cm−1). Some fluorine-free anions, such as perchlorate (ClO4−), dioxalate borate (known as BOB), trioxalate phosphate, tetracyanoborate, and dicyanotriazole salts have been developed. The main challenge of these non-fluorinated salts is their poor ability to passivate aluminum fluid collectors at high potentials. In fact, one reason LiPF6 has traditionally been used in lithium-ion batteries is its ability to passivate aluminum, thanks largely to its fluorine atoms.
Common non - fluoride additives are vinyl carbonate (VC), tri (trimethylsilane) phosphite (TMSP). LiBOB and LiNO3 are also available as fluorine-free additives at low concentrations. If the formation of stable SEI is not considered, more additives can be incorporated into the selection.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
