Starting from the characteristics of the electrolyte, the problem of fast charging of lithium-ion batteries has made a new breakthrough. On February 29, the team of researcher Fan Xiulin of the School of Materials Science and Engineering of Zhejiang University, in cooperation with domestic and foreign researchers, published a paper in the international journal Nature - the research team designed a new electrolyte that can support high specific energy lithium-ion batteries in the ultra-wide temperature zone of -70 ° C to 60 ° C for reversible charge and discharge, and fast charge and discharge at room temperature.

Lithium-ion soft-pack batteries in research. Research team for the picture
During the study, the research team developed and validated a new set of extreme electrolyte design principles that broke the traditional lithium-ion transport mode and determined the optimal formulation of the new electrolyte from tens of thousands of solvents. The test data show that the ionic conductivity of the new electrolyte is 4 times that of the commercial electrolyte at 25℃ and 3 orders of magnitude higher at -70℃.
According to the researchers, the cost of this new electrolyte preparation battery is still high, but it can be first applied in extreme temperature situations such as polar research, space exploration, and seabed exploration.
Details of the thesis are as follows:
With the application of lithium-ion batteries gradually to electric vehicles, seabed exploration, space exploration, polar research and other fields, it is required that lithium-ion batteries have high energy density, high rate and wide temperature range stable circulation, which requires the electrolyte must have high ion conductivity, low solvation energy and wide liquid range, and form a stable interface film in the positive and negative electrodes. This is not easy to achieve with current electrolytes. The interaction between solvent-Li-ion and anion determines the properties of the electrolyte, including ionic conductivity, solvation/desolvation behavior, interfacial chemistry, etc. Among them, high ionic conductivity requires high lithium ion solvation energy, while anion-derived interface films require low lithium ion solvation energy, and it is difficult to meet the above two characteristics based on the current electrolyte design principles. How to adjust the solvent-Li-ion - anion interaction so that the electrolyte has both fast ion transport dynamics and stable and effective interface film is still a challenge in the current electrolyte design.
In order to achieve the stable circulation of lithium-ion batteries under harsh conditions, the team of Professor Chen Lixin and researcher Fan Xiulin from the School of Materials, Zhejiang University, together with Professor Wang Chunsheng from the University of Maryland and Professor Hu Enyuan from Brookhaven National Laboratory, proposed the design principle of fast ion transport electrolyte and established a unified framework for the transport behavior of lithium ions in liquid electrolyte and solid electrolyte. A "ligand channel facilitated transport" mechanism was proposed and validated to accelerate the transport of lithium ions, thus achieving a stable cycle of high rate (≥6C), wide temperature range (-70°C~60°C) and high specific energy lithium-ion batteries.
The research results were published online by the international top journal Nature on February 29, 2024 (Beijing time). The first authors are Lu Di, PhD candidate, and Li Ruhong, Researcher, Zhejiang University; corresponding authors are Fan Xiulin, Researcher, Zhejiang University; Wang Chunsheng, Professor, University of Maryland; and Hu Enyuan, Professor, Brookhaven National Laboratory. It was supported by Professor Chen Lixin, Associate Professor Fan Liwu and Associate Professor Xiao Xuezhang of Zhejiang University, as well as Researcher Wang Jianping of the Institute of Chemistry of the Chinese Academy of Sciences and Dr. Deng Tao of the University of Maryland (now Associate Professor of the Sino-UK Low Carbon Research Institute of Shanghai Jiao Tong University). Zhejiang University is the first communication unit of this paper.
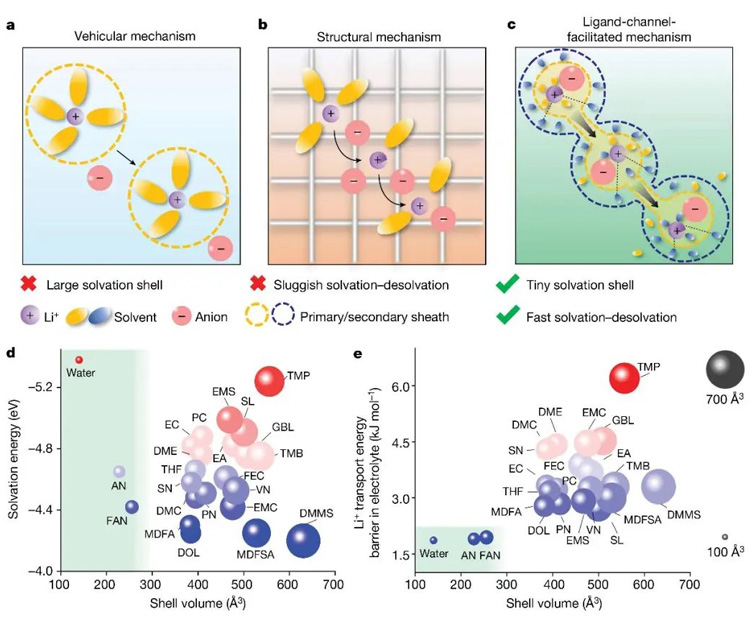
Figure 1. Electrolyte design principles and solvent screening strategies. (a) Media transmission; (b) Structural transmission; (c) Ligand channels facilitate transmission; (d) The relationship between solvation energy of lithium ion and solvation sheath size; (e) Relationship between lithium ion transport energy barrier and solvation sheath size.
The research team screened 23 solvent materials based on the solvation energy of lithium ions, the size of the solvation sheath and the transport energy barrier of lithium ions, among which three solvents, water (H2O), acetonitrile (AN) and fluoroacetonitrile (FAN), were screened. Considering that H2O and AN solvents do not form a stable solid electrolyte interface film (SEI) at the negative electrode interface, only FAN meets the requirements of electrolyte under harsh conditions. The traditional transmission mode of lithium ions in electrolyte can be divided into two types: dielectric transmission and structural transmission, but the electrolyte design based on both of them is difficult to meet the needs of rapid transmission of lithium ions in electrolyte. The research team further proposed a mechanism of "ligand channel promoting transport" with small molecule solvents, through which small molecule solvents in the outer solvation sheath interact with the inner layer of lithium ions to form a lithium ion transport channel, reduce the ion transport energy barrier, and accelerate lithium ion diffusion (Figure 1).
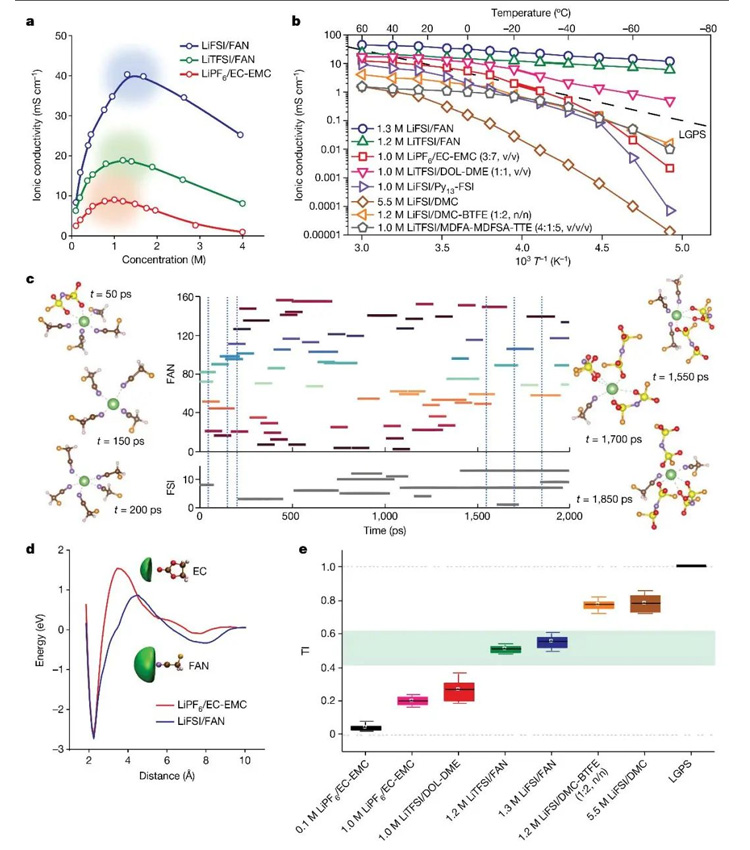
FIG. 2. Properties and ion transport mechanism of FAN-based electrolyte. (a) ionic conductivity of electrolyte at different concentrations; (b) ionic conductivity of the electrolyte over a wide temperature range; (c) Molecular dynamics simulation of lithium ion solvation environment in electrolyte at room temperature; (d) the change of lithium ion transition energy barrier in different electrolytes; (e) TI values of different electrolytes.
Based on the above criteria, the research team designed a new electrolyte [1.3M lithium difluorosulfonimide (LiFSI)/FAN] that achieves a wide temperature and high ionic conductivity (25°C: 40.3mS /cm,-70°C: 11.9mS /cm) and generates LiF-LixN-rich SEI. In order to further study the transport mechanism of lithium ions in the electrolyte, the research team proposed a "transport index (TI)" to quantitatively describe the ion transport behavior in the electrolyte. In ideal dilute solution (TI=0), the transport mode of lithium ion is medium transport. In the solid electrolyte (TI=1), the transport mode of lithium ion is structural transport. When the electrolyte has a ligand channel promoting transport mechanism and TI is about 0.5, the ionic conductivity reaches the maximum value, achieving a unique and fast ion transport behavior different from the medium/structure transport. It is worth noting that TI as a direct and effective parameter can guide the design of ideal electrolytes with superionic behavior (Figure 2).
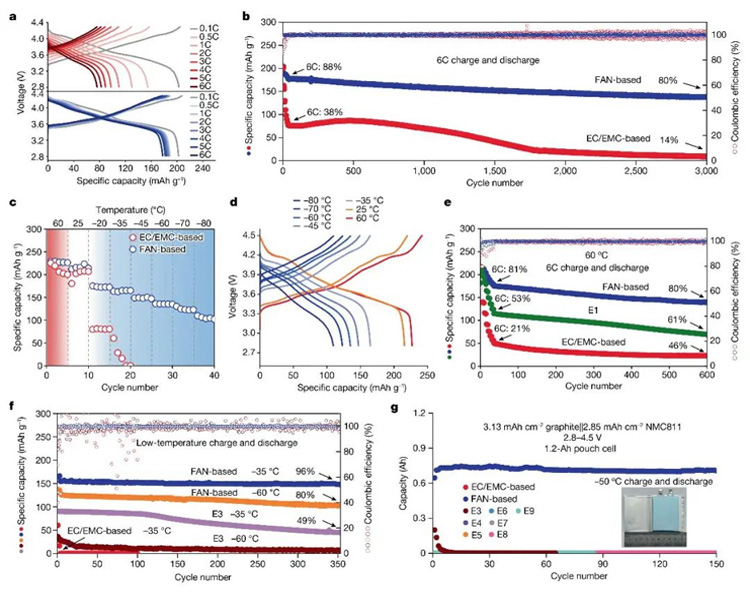
FIG. 3. Electrochemical performance of FAN-based electrolyte. (a-b) room temperature rate performance; (c-d) wide temperature performance; (e) high temperature magnification performance; (f-g) Low temperature performance.
Thanks to high ionic conductivity and interfacial passivation capability, 1.3M LiFSI/FAN electrolyte achieves high rate (≥6C) stable cycling at room temperature and high temperature. It is worth noting that for graphite-based lithium-ion batteries, low temperature charging is particularly challenging, and the electrolyte designed by the research team can achieve a 4.5V graphite ||NMC811 battery with a reversible charge and discharge cycle of more than 350 cycles at -35°C, and the capacity retention rate is still as high as 96%; At the same time, the 1.2Ah 4.5V graphite ||NMC811 soft pack battery can be reversibly charged and discharged for more than 150 cycles at -50°C without significant capacity attenuation (Figure 3).
In addition, the research team showed by HRTEM, XAS and theoretical calculations that FAN solvent and FSI-anion would decompose at the negative electrode interface to form LiF-LixN-rich SEI. This low-impedance and efficient interface film facilitates the rapid transport of lithium ions (Figure 4).
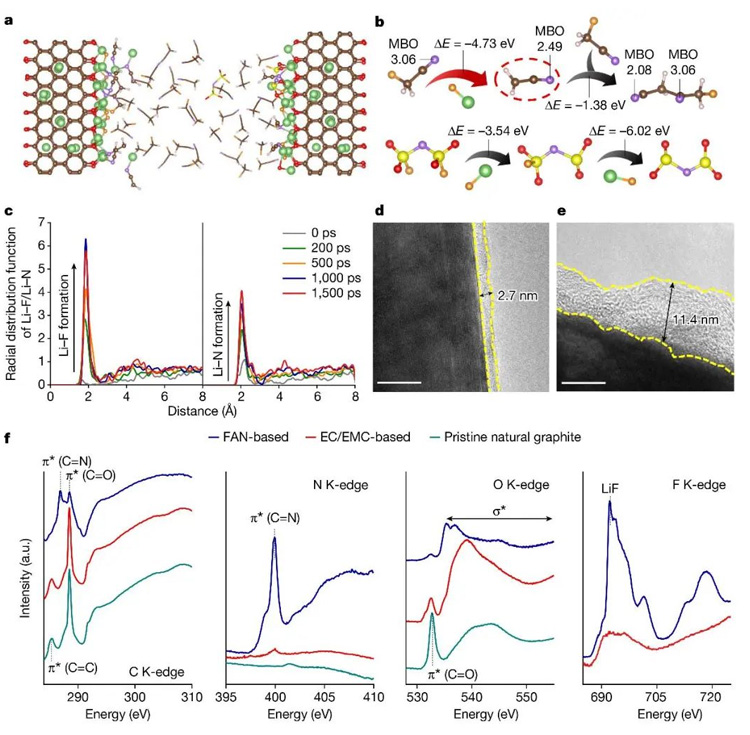
Figure 4. Analysis of FAN-based electrolyte/electrode interface. (a) Simulation of the initial decomposition reaction of the electrolyte at the negative interface; (b) multi-step decomposition pathway of FAN solvent and FSI-anion; (c) Radial distribution function; (d-e) HRTEM diagram of graphite negative electrode after circulation in different electrolytes; (f) XAS diagrams of the recycled graphite anode and the original graphite electrode in different electrolytes.
Through the in-depth investigation of the ion transport mechanism, this study proposed the selection descriptor of fast ion transport solvent and the design principle of electrolyte, which provided an important theoretical basis and technical exploration for the development of high specific energy lithium-ion battery electrolyte under harsh conditions.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
